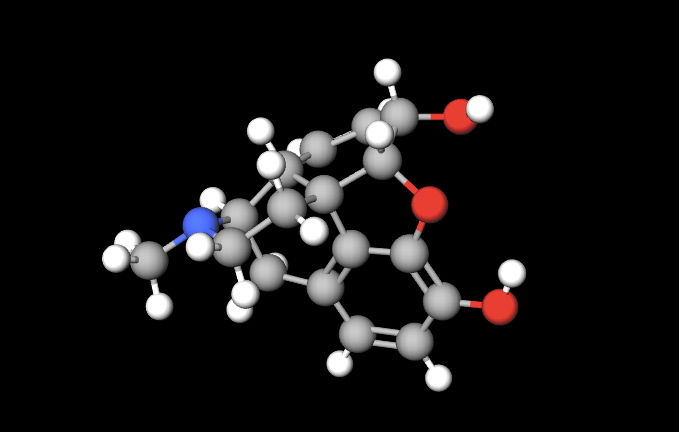
I’ve started reviewing for the MCAT and have begun by studying what I learned in Gen Chem. This afternoon, I read a chapter on the mechanisms by which atomic bonding contributes to the shape of a molecule, and how modern medicine takes advantage of this. Many drugs work by a process not unlike a lock and key. One molecule binds to another in just the right way, producing a cascade of reactions that deliver the desired physiological effect. When chemists are able to manufacture a synthetic chemical that mimics a naturally occurring one, (similar to copying a key that fits a specific keyhole), some pretty amazing things can happen. A great example of this all began when the German chemist Friedrich Sertürner isolated a chemical sometime between 1803-1805 and named it after the Greek god of dreams. The effects of morphine have been utilized for thousands of years by many civilizations. Until Serturner however, never had the active ingredient been used extensively as an anesthetic. Nicknamed “God’s Medicine,” morphine quickly became popular as the silver bullet to alleviate pain. This is a model of the actual molecule Serturner isolated.
Morphine works by binding to opiate receptors on cells within our nervous system. A curious question then arises. Why do our bodies’ cells have receptors for a chemical produced by plants? Scientists wrestled with this question for years and many believed there was a substance produced by our own bodies that would bind in the same receptors and therefore generate a similar effect. It wasn’t until the 1970’s when a few of these would be discovered an named endorphins. The term endorphin actually stems from the combination of endogenous (within living organisms) + morphine = endorphin. Isn’t it interesting that a compound found to be produced naturally in our bodies was named after that discovered first in a plant? When Morphine binds to opiate receptors in nerve cells, signal transduction is interrupted.
The understanding that structure determines properties is a fundamental concept in chemistry. Understanding the structure of tiny particles such as atoms, allows us to see how molecules form and how they, in addition, react with others. This way of thinking has transformed every field of science, especially those in biology. I’ll probably make some more posts about how certain common drugs work as I find it super fascinating. The concept that shape determines many of the characteristics of a molecule is an important one and anyone interested in learning more should combine this with a study of VESPR (valence electron shell repulsion) theory, and molecular orbital theory. I’m continually amazed by what has been discovered, how much we know, and what feats are possible when we apply that knowledge.